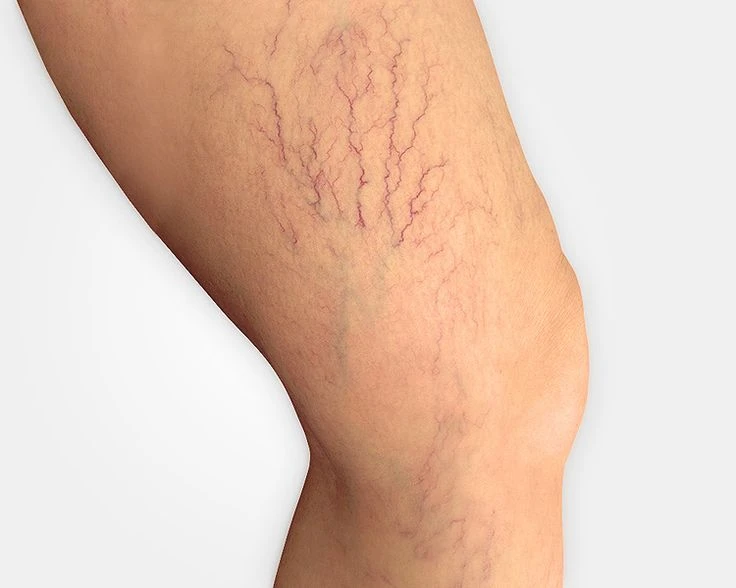
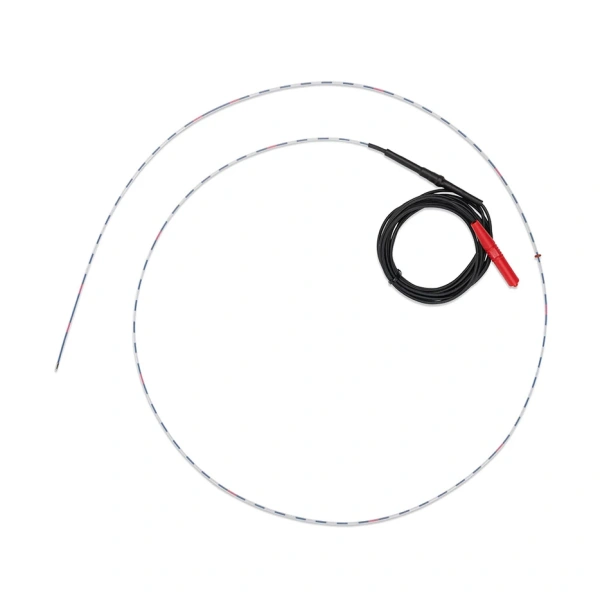
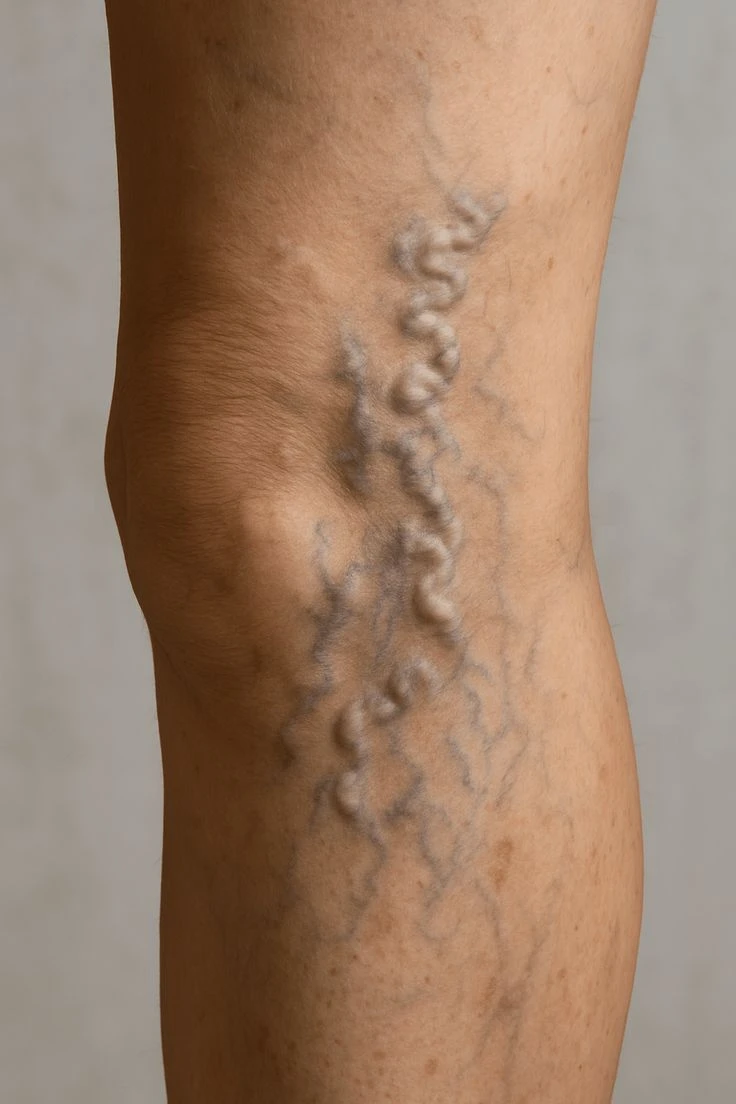
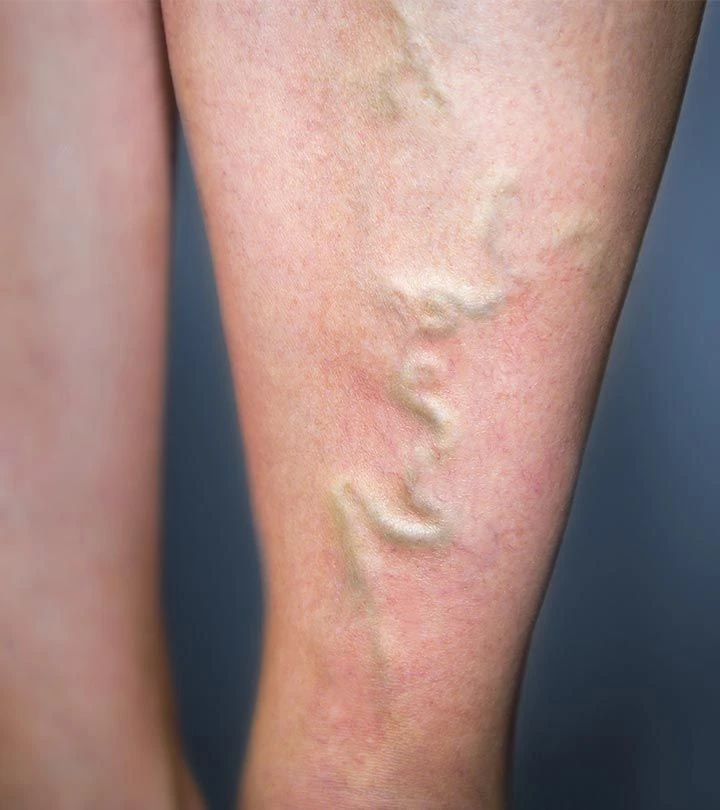
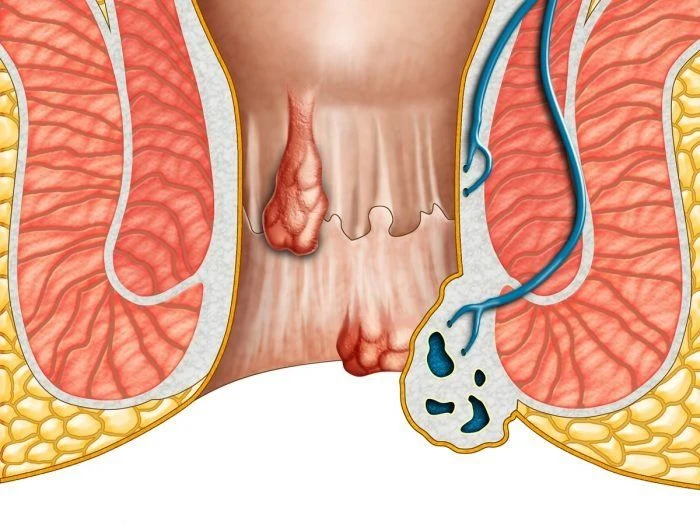
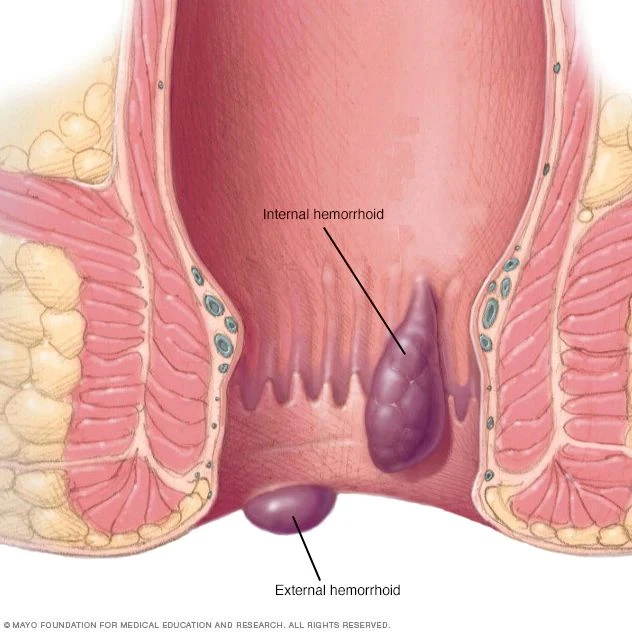
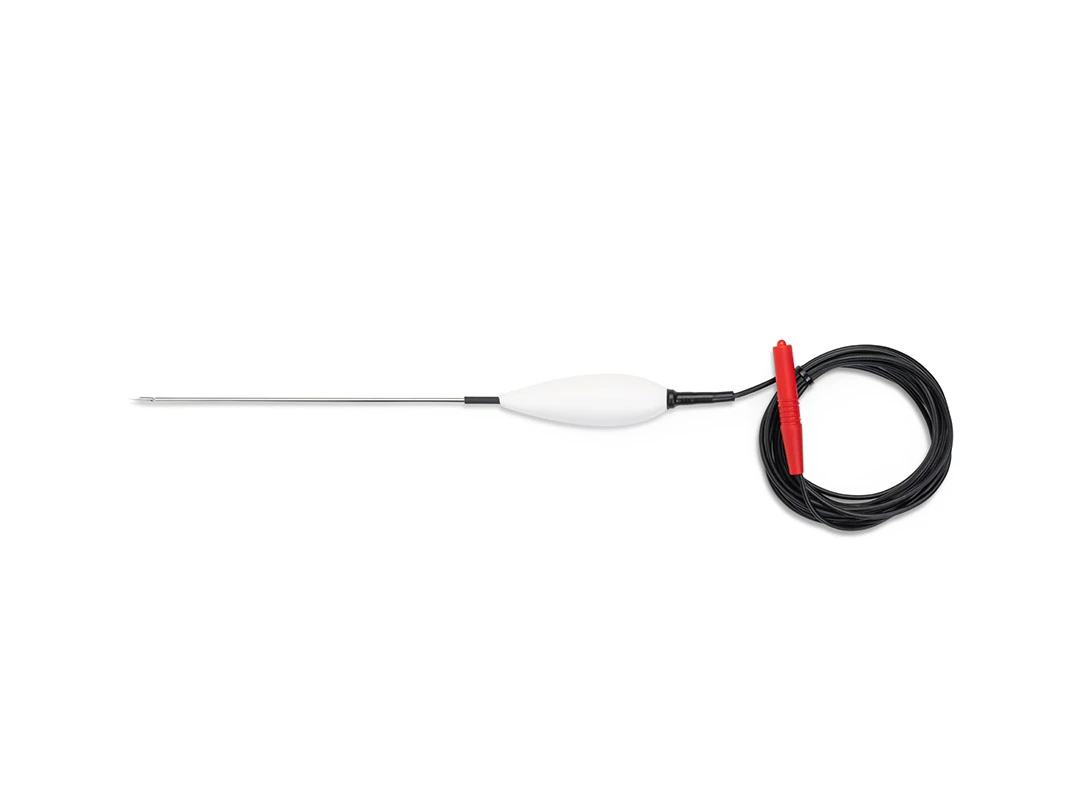
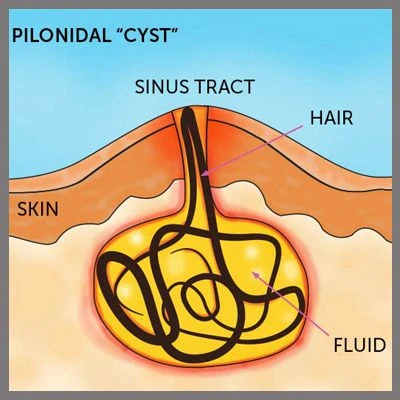
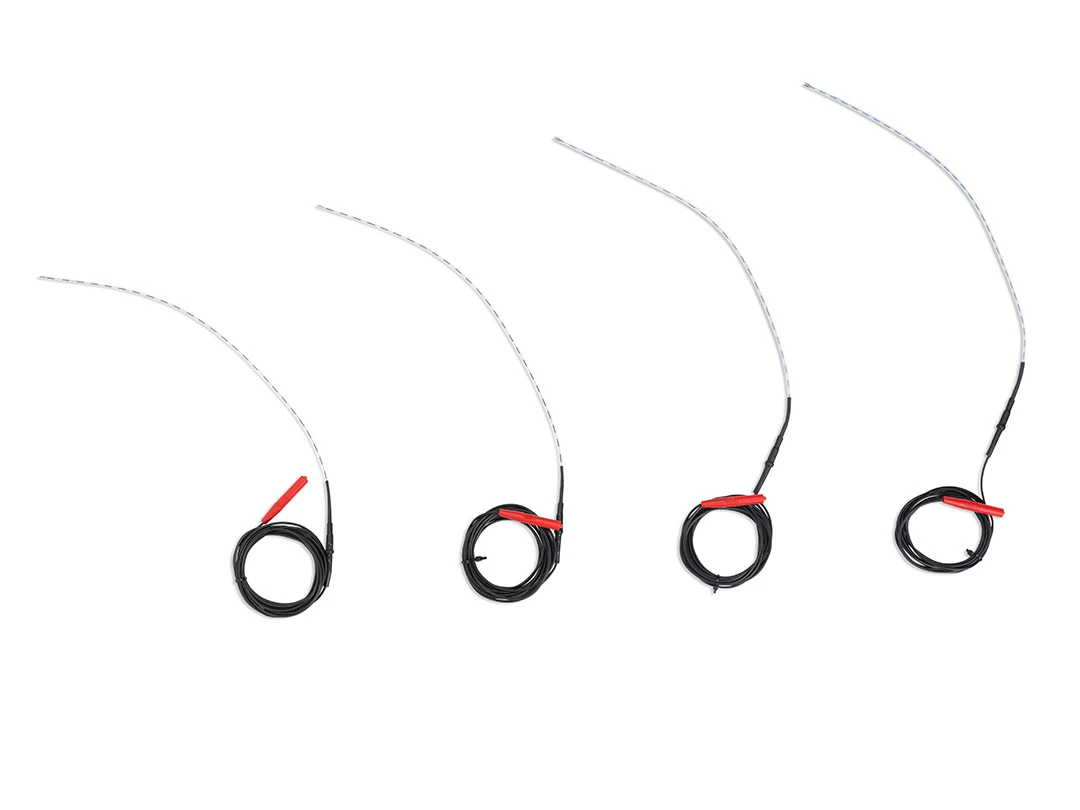
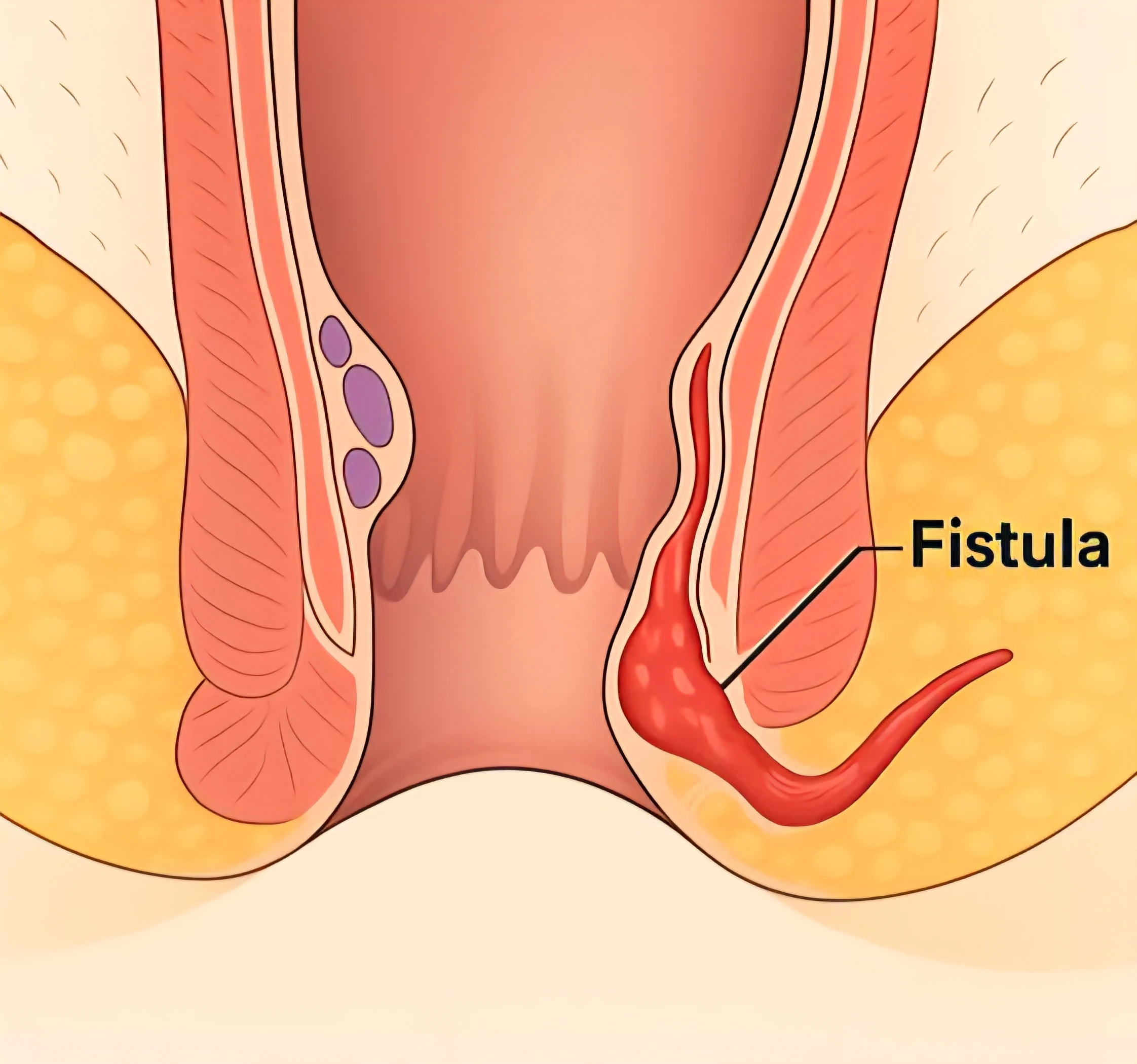
The EVRF device, manufactured by FCare Systems in Belgium, utilizes thermocoagulation technology and radiofrequency waves. With its specialized catheters and probes, it is designed for use in two medical fields: Proctology and Phlebology. In the following sections, we will take a closer look at the device and its different types of catheters and probes.

Thermal Coagulation
All EVRF treatments are based on the principle of thermocoagulation. This principle involves heating the vein walls by transmitting a signal through a catheter or needle, which causes the cells to vibrate and the temperature to rise.
Thermal coagulation with radiofrequency is carried out in four stages:
- Cell ionization
- Tissue evaporation and dehydration
- Denaturation of collagen fibers
- Vasculazation of the innermost vessel membrane
Specifications
110-230 V / 50-60 Hz
125 VA
IPX0
class I
2 x F2A / 250 V
BF type device
Class IIa device
Output of the thermocoagulation (HF) signal
Temporized fuse in glass
Class IIa device
Output of the thermocoagulation (HF) signal
Temporized fuse in glass
Device Dimension
120 mm
280 mm
360 mm
12 kg